Day 1: Molecular Geometry and Polarity
To warm up for the lesson, have students write descriptions or draw diagrams to illustrate the difference between a compound and a molecule. Have them fill in the blanks with “compound” or “molecule” in the following sentence: “A _____________ is always a _____________, but a _____________ is not always a _____________.” (A molecule is always a compound, but a compound is not always a molecule.)
Mini-Lesson
Tell students the objectives for this lesson. Present a mini-lesson about molecular geometry, based on the following information.
Molecular polarity depends on two factors: the difference in electronegativity between atoms in a compound, and whether the compound’s structure is symmetrical. When you have atoms of more than two elements in a compound, you cannot use electronegativity differences to predict whether the compound is polar. Instead, you have to use the molecule’s geometry (three-dimensional shape) to predict the polarity.
Molecular geometry is important because it is responsible for the following properties of a substance:
- reactivity
- polarity
- state of matter
- color
- magnetism
- biological activity
There is a theory that explains how the polarity of molecules affects their three-dimensional shape. It is called the valence shell electron-pair repulsion theory, or “VSEPR” (pronounce “vesper”) for short. VSEPR tells us that the valence electron pairs surrounding an atom repel each other, and will take an arrangement that minimizes this repulsion. This determines the molecular geometry. To determine the number of electron pairs in the valence shell of a central atom, you can draw the Lewis structure of the molecule, showing all non-bonded and bonded pairs of electrons.
Tell students just as chemical bonds can be polar, molecules can be polar. Polar molecules are called dipoles. Explain that we can use illustrations of molecules to determine if they are polar.
In the water molecule shown below, the two lone electron pairs on the oxygen atom create a negative pole on the molecule, and the electrons bonded to the hydrogen atoms make a positive pole. Molecules can also contain polar bonds, but not be polar. In the diagram below, both of the C–O bonds in carbon dioxide are polar, but they are opposite from each other, so they cancel each other out and the molecule overall is nonpolar. Have students copy the diagrams below into their notes.
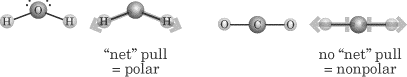
Source: http://img.sparknotes.com/content/testprep/bookimgs/sat2/chemistry/0002/sat117002_0437.gif
Give students copies of Molecular Geometry Examples and display it with an overhead projector or document camera (S-C-6-3_Molecular Geometry Examples.doc). Use the examples to show students how the lone electron pairs and bonded electrons repel each other to determine the geometry of the molecule. It may be helpful to explain that it is like having magnets with the same poles facing each other, and that like repels like. Explain why each of the examples is polar or nonpolar:
- CO2 is nonpolar because the opposite polar bonds cancel each other out (as explained above).
- HF is polar because the two elements have different electronegativities, so they pull unequally on the electrons.
- BF3 is nonpolar because all of the atoms lie in the same plane, and the fluorine atoms have an equal pull on the electrons from the boron atom.
- NO2 is polar because the nonbonded electron pair on the nitrogen atom repels the electrons that are bonded with the oxygen atoms; the electrons are unequally shared between the atoms.
- NH3 is polar because the nonbonded electron pair on the nitrogen atom repels the electrons that are bonded with the hydrogen atoms; the electrons are unequally shared between the atoms.
- CH4 is nonpolar because the four hydrogen atoms all have an equal pull on the electrons that are bonded with the carbon atom; there are no nonbonded electron pairs.
Guided Practice
Give students the Predicting the Polarity of a Molecule worksheet (S-C-6-3_Predicting the Polarity of a Molecule and KEY.doc). Explain how each of the formulas can be used to predict the polarity of a molecule. Give students a few minutes to work on the chart, and then complete the chart as a whole-class activity, with students volunteering the answers.
In pairs, have students complete the Molecular Shapes Practice worksheet (S-C-6-3_Molecular Shapes Practice and KEY.doc).
Assign the Molecular Shapes worksheet (S-C-6-3_Molecular Shapes Worksheet and KEY.doc) for homework.
Day 2: Building Molecular Models with Molecule Kits
Begin the class by having students write an answer to the question, “How can we predict polarity based on illustrations of molecules?” We can predict polarity by looking at the bonded electrons and lone electron pairs to see if they are symmetrical (nonpolar molecule) or asymmetrical (polar molecule). Have several students share their answers.
Divide students into small groups, depending on the number of molecular model kits you have available. If students have not used the kits before, demonstrate how to construct a few molecules with the kits. Have the groups construct each of the molecules from the Molecular Model Examples handout, based on the illustrations on the worksheet. As they complete each model, the groups should raise their hands so that you can check the model and have them explain why the molecule is polar or nonpolar.
Extension:
- Using each molecule on the Molecular Geometry Examples handout, students who might need an opportunity for additional learning can practice drawing Lewis dot structures to better see how the lone electron pairs play a role in molecular shape.
- Have students create a key for themselves with definitions of each of the following terms that are used to describe molecular shape: bent, linear, planar, tetrahedral, and trigonal.
- Students who may be going beyond the standards can research and build models of exceptional molecular configurations (e.g., seesaw, octahedral, and T-shaped).
- Students who may be going beyond the standards can do the interactive module for determining the polarity of molecules at http://employees.oneonta.edu/viningwj/sims/determining_molecular_polarity_m.html.
- Have students read about resonance structures, then construct models to show the resonance structures of a compound such as NO2, NO3, or CO3. See http://cost.georgiasouthern.edu/chemistry/general/molecule/resonan.htm for more information on resonance structures.
